In this interview, we explore rapid on-site evaluation and the need for process enhancement.
MC: I’m Mark Clymer, Vice President of Product at ACCU-SCOPE, and we’re here to learn about ROSE and ASP Health‘s vision to improve ROSE outcomes. Joining us today is Dr. Hari Subramanian, Executive Vice President and the Chief Commercial Officer at ASP Health. Welcome Hari.
HS: Thank you, Mark. It’s definitely a great pleasure to talk about rapid on-site evaluation – ROSE for short – as well as what ASP Health is doing in the larger context of the overall rapid on-site evaluation market.
MC: Hari, can you give us a picture of the landscape. What is ROSE and what are the current challenges facing healthcare facilities?
HS: That’s a great question. Rapid On-Site Evaluation is not a new concept within cytology. We were surprised to learn that the initial papers on rapid on-site evaluation date back as early as 1990’s and early 2000’s when people were applying this concept of rapid on-site evaluation.
First, let’s talk about what ROSE is, and why did rapid on-site evaluation even come about? Let’s say, for example, a patient goes in with a tumor in their thyroid or in their lung. They go to the physician, but they don’t know what kind of a tumor it is. It could be a benign tumor, some kind of infection, or it could be a cancer. The physician needs to look at the cells in these tumors in order to make a diagnosis. If it’s a thyroid, an endocrinologist or an interventional radiologist would look at the thyroid tumor, and take a sample from there. If it is lung, an interventional pulmonologist would take a sample from down in the lung. The tumor samples are collected and sent to the lab. It’s as easy as that, right? It turns out that 30 to 40% of the samples sent to the lab for diagnosis, unfortunately, contain no material or very limited material to make a diagnosis. From the patients’ perspective, they have done everything they’ve been told to do, and now they wait for the results. But for about 40% of them, they get a call saying, “You have to come back in to give more sample.” Now the patient must go through the same process again. Think about the psychological issues — the patients are already tense, they are freaking out, and they get the call from the doctor that, okay, you know what? You need to come back. Also think about it from the hospital’s perspective. These procedures are getting repeated, taking the physician’s time, the hospital’s time and the patient’s time, etc.
This is the reason why the cytology and pathology community came together with the interventionists, and developed this particular concept of rapid on-site evaluation. As the name suggests, very quickly they can look at the samples coming from these tumors to evaluate whether there is enough material in the sample for diagnosis. If there is enough material, the patient goes to recovery, and if there isn’t enough material, the doctor can collect another sample right then and there, while the patient is in the procedure room, confirms the presence of material using ROSE, and then sends the samples to the lab.
It started as an adequacy assessment, and that’s why you hear it called rapid on-site evaluation or adequacy assessment. For those patients who go through FNAs without rapid on-site evaluation, unfortunately, they’re the ones who must come back again. It’s a nightmare for them. You can imagine why that would be the case.
Now the question is, “Why is ASP Health trying to do what we are trying to do?” What are the challenges with rapid on-site evaluation? As with many issues in healthcare that we are currently facing, there is a huge shortage of personnel, period. You constantly hear about the shortages of nurses, waiting times in emergency rooms, etc. The same thing is happening within cytology and pathology as well. There is a huge shortage of cytotechnologists, the people trained to perform rapid on-site evaluation. Of course, there is also a shortage of pathologists or cytopathologists, the physicians who make the decisions. In fact, many schools that used to offer cytotechnology degrees closed during the pandemic and have not reopened their programs. Currently in the US, there are only a few institutions who even offer cytotechnology degrees and training.
What happens now? One of the two things happen. First, the number of people trained to perform rapid on-site evaluations has come down dramatically, and it continues to decrease. Second, and the flip side of the first, the number of patients needing these types of FNA procedures, and therefore rapid on-site evaluation, is constantly increasing. Consider that in a large clinical site that has enough cytotechnology professionals to perform rapid on-site evaluation, not every cytotechnologist performs at the same level. Cytotech #1 may perform amazing rapid on-site evaluation, while Cytotech #2 might be new and may not perform rapid on-site evaluation in a similar manner. This tension is always present. There is a lack of standards, it’s highly time-consuming, and it’s a completely manual process.
Cytotechnologists are trained to read the cytology slides from pap smears or other body fluids, and this is the majority of their task in the lab. However, now they are being forced to spend much of their time in the procedure rooms and stand shoulder to shoulder with the physicians as part of this rapid on-site evaluation. With staff shortages, all these things create a huge nightmare situation for the rapid on-site evaluation itself.
Adding fuel to the fire, there is limited reimbursement for rapid on-site evaluation. The cytotechnologist goes into the procedure room and performs rapid on-site evaluation. You would think they’ll be reimbursed, that there is a very good CPT code. Unfortunately, there is no reimbursement, UNLESS you have a cytopathologist directly involved with the rapid on-site evaluation.
To summarize, you have non-standardized techniques, high variability due to the manual nature of the process, it’s highly time-consuming, limited reimbursement, and a huge staff shortage. Sure, some big sites like Mayo or Cleveland have the staff to perform rapid on-site evaluation within their FNA clinics, but small regional or community hospitals do not have these resources. Disruptive technology is needed, one that democratizes the entire rapid on-site evaluation process. That’s a long-winded answer to a simple question, but I wanted to cover the bases in this big ballpark.
MC: Thank you for that background. There seems to be a systematic failure despite having the technology and the training. A failure in providing the level of care or diagnostics that we should be capable of.
ASP Health developed some products and some techniques in sample preparation. How did ASP Health choose the mission to improve ROSE results? Was this your goal from the start? Can you explain to us how ASP Health chose to get into the ROSE performance business?
HS: I’d really like to say that we did an amazing job with market research, created a market flow map, etc., but that wouldn’t be true. In this case, we had an “aha” moment. My colleague and I visited a clinical site to get the voice of the customer. At that time, ASP Health was trying to find a market need for our initial product. We watched them perform rapid on-site evaluation, and we said to ourselves, “we need to improve this process.”
So what did we see? We saw an endocrinologist collect samples from the patient. A cytotechnology supervisor was also in there – these are senior cytotechnologists with more than 20 years of training. Then they start using these manual methods. They take the sample from the patient, put a couple drops onto a glass slide, and manually smear it. They then use a hair dryer to dry the slide, then do this dip and dunk process to stain the slide. As the cytotechnologist is doing this, the next sample arrives from the physician. The cytotechnologist has to literally drop the slide into the stainer, fetch the new slide from the physician, and repeat the entire process. During this time, the cytotechnologist must take care of the first slide, and look at it under the microscope, all while being barked at by the endocrinologist. “What is the result? Give me the result right now. Is there enough material or not? I want to get done with this patient.” This was a huge discovery, and this is how ROSE is still being done in many places. Realize that the patient never sees any of this process either – they’re under anesthesia. That was the aha moment for us – this needs to change. Our thought was that a completely automated solution could change the workflow for the cytotechnologist, and improve the interaction between the endocrinologist and the cytotechnologist, among other things.
MC: Besides the manual procedure, the dynamics of the interaction was an eye-opening experience. What steps has ASP Health taken to make the ROSE procedure more efficient? From your explanation, there are multiple steps involved, right?
HS: As a first step, ASP Health partnered with a product development firm to develop the first instrument called ROSE Prep™. ROSE Prep automatically prepares specimen slides for rapid on-site evaluation. We collaborated with many cytotechnologists and cytopathologists at top clinics to develop and test this early ROSE Prep product before commercializing it. The goal was to fully automate the process, eliminating the manual preparation. No more dip and dunk. Just get the sample from the patient, load it into the machine, and with the click of a button, the slides are automatically made within 70 seconds. ROSE Prep relieves the pressure of preparing the slides, and the cytotechnologist can instead focus on reading the slides. ROSE Prep was the first product we launched, and we’ve gotten a lot of positive feedback on it.
But ROSE is more than just preparing the specimen slides. It involves slide preparation, reading the slides, and providing actionable feedback to the physician – the endocrinologist, interventional radiologist or interventional pulmonologist. What is actionable feedback? If the first sample taken by the physician only contains blood and no material, the physician can reposition and poke another area to collect a sample. ROSE is repeated. Now the sample has material in there. The physician is told that there is material, and they collect two more samples from the same area. Then the procedure is done. This is how the feedback loop actually works.
We learned that, although customers love ROSE Prep with its automated sample preparation, cytopathologists still must go down to the procedure room. Specifically in small regional centers, cytopathologists don’t have time to go down, wait for the specimens, etc. They are looking for a solution to avoid the travel to the procedure room, so a fully automated microscope is a reasonable solution and allows them to look at the slides remotely.
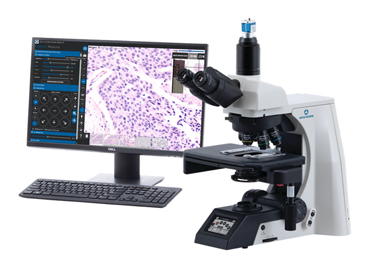
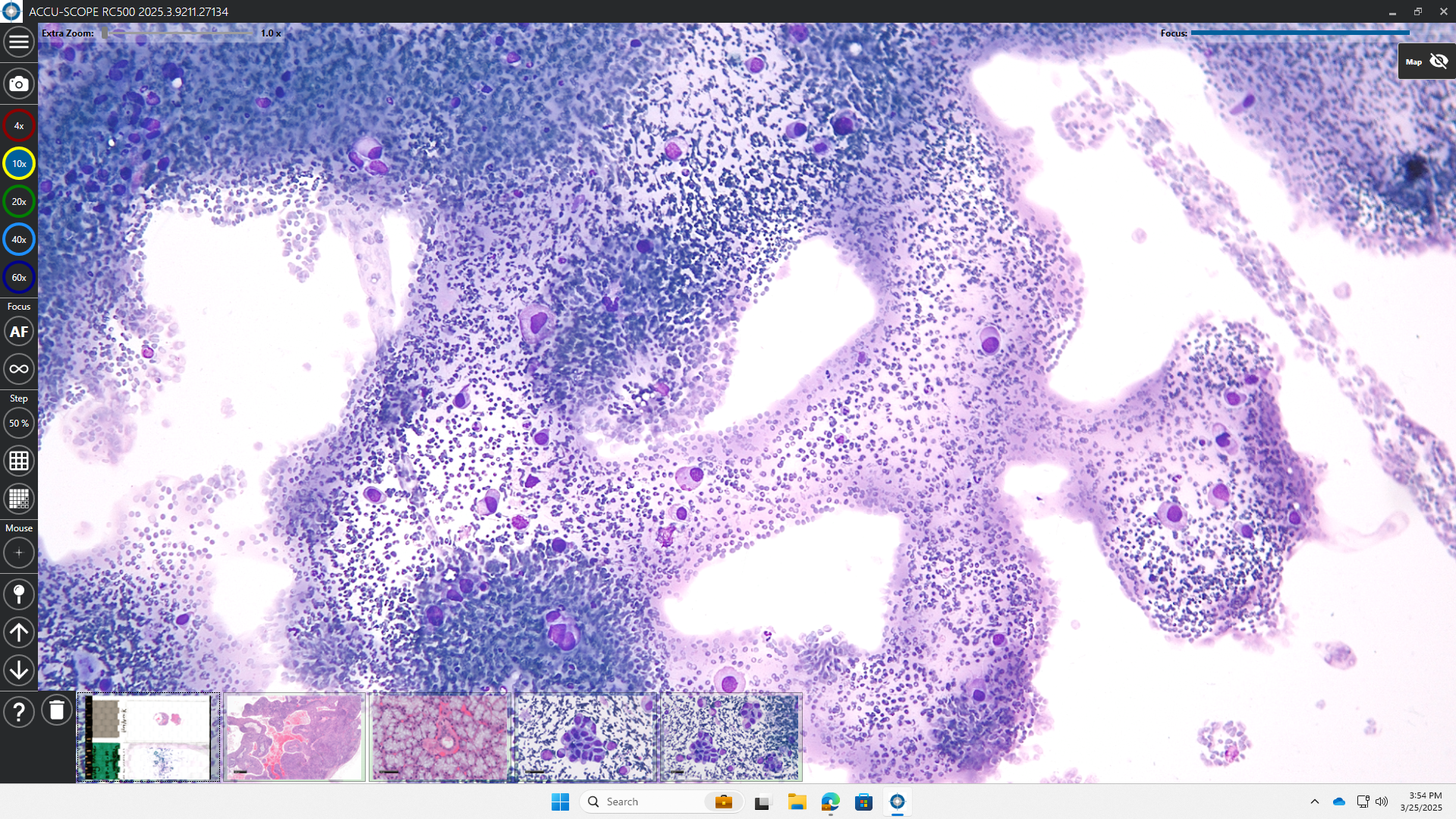
After looking at existing and custom technologies, we found that ACCU-SCOPE’s RC500 system was a good fit for our customer expectations. The RC500 enables a full remote control of the microscope. The cytopathologist doesn’t have to be physically present in front of the microscope to view the samples. The cytotechnologist prepares the slides using ROSE Prep and loads them onto the RC500. The cytopathologist can remotely log into the system from their office and control the microscope as if they were sitting right there. So now we can combine both ROSE Prep with ROSE View™, our remote viewing system that includes this RC500 microscope. This combination solution saves tremendous time and, possibly more importantly, staff cost. Even though the pathologist reviews the ROSE slides remotely, they still get reimbursement for these ROSE procedures.
This is how ASP Health is trying to achieve an efficient, rapid on-site evaluation process, compared to the status quo of a manual process.
MC: It sounds like a winning combination. ROSE Prep for slide preparation and ROSE View for remote control and viewing of the specimens to determine sample adequacy before the sample goes to the laboratory for traditional preparation and staining.
At the recent American Society of Cytopathology conference, you had a chance to show and demonstrate ROSE Prep and ROSE View. How do you get the word out about these solutions, that there is an alternative to traditional manual process?
HS: We are doing two things, one from the product perspective and the other from the marketing perspective. From the product perspective, we are offering the opportunity to purchase both the ROSE Prep instrument together with the ROSE View system. For the moment, our customers must use a mouse and keyboard to examine these slides remotely. ASP Health is using its massive repository of specimen slides prepared using our own ROSE Prep instrument, together with machine learning, to very quickly identify areas where the cells are present on these slides. We call this ROSE Assist™. The purpose of this machine learning software is not to make decisions for the physicians, but to point them to the location of cells so that they can use the remote microscope to view those areas and make an adequacy assessment from there.
We showcased these three different products at the ASC conference: ROSE Prep, ROSE View and ROSE Assist. Many cytopathologists and cytotechnologists were thrilled to see them for a variety of reasons. Many people see this as a game changer. For the first time, they could see a complete end-to-end ROSE solution that will help them perform ROSE (including automatic sample preparation, remote viewing, and quick cell location detection) without the headaches of the past. For this advancement, ASP Health was awarded “most innovative cytology practice” by the American Society of Cytopathology at the recent meeting in Orlando.
Now, from the marketing perspective, we recently launched a campaign called “De-Stress Your ROSE.” For many physicians, ROSE is a very stressful process – they want to perform rapid on-site evaluation, but they want to avoid the challenges we discussed earlier. The De-Stress Your ROSE campaign communicates that there is a solution, or set of solutions, available to help them de-stress their overall ROSE process and still offer their customers and patients the best care possible. So, that’s a summary of our current initiatives.
MC: This campaign sounds like a public service announcement targeted to cytopathologists where they not only recognize the benefits of ROSE, but they also recognize the stresses and disruptive nature that it brings. Acknowledgement is the first step. ASP Health provides the end-to-end solution that not only prepares the slides but also extends the process to assist the cytopathologist in their evaluation of the samples. What are your expectations for the De-Stress Your ROSE campaign? Is there involvement or interest from the ASC to see a reduction in the cost of ROSE procedures, the duration of ROSE procedures, or some other performance metric?
HS: We’re still an early-stage company and don’t have a huge marketing budget to push this De-Stress Your ROSE campaign. We have a two-year time horizon to fully educate our customers on ways to de-stress their ROSE. For us, it is a long-term commitment, and we want to continue to provide innovative solutions for our customers.
Our internal KPIs are closely linked to some of the challenges that we are facing, such as the adoption of these new technologies like ROSE. Change management is important because we are asking them to change the way they’re already performing ROSE. We have to slowly shift that mindset. Customers don’t come to these conferences to solve problems with ROSE – they’re not aware of any technologies that provide solutions for ROSE. Finding a solution for ROSE is not at the top of their mind. It’s when they visit our booth, see the solutions, and think, “Wow. This is interesting.” But when they return to work, the excitement is lost. Our goal over the next year is to educate 20-25% of our customers that there are problems and that there is a solution available for rapid on-site evaluation.
The second KPI also relates to solving a challenge. Many customers don’t know that the ROSE problem can be solved, and they give up on it or just accept it. About five years ago, they would have been performing ROSE on many patients, but because of accelerated staff shortages post-COVID, they say, “I’m not going to perform ROSE anymore.” Let me give you a scenario of progress. Five years ago, I did not know anything about ROSE or its importance while performing an FNA. Flash forward five years and, with education, my perspective has changed. The first question I’m going to ask the physician is, “Are you going to perform rapid on-site evaluation for this particular Fine Needle Aspirate process?” Once I know about ROSE, I want to have ROSE. The problem is that patients are not aware of the need to perform or benefits of rapid on-site evaluation. Many publications show the importance of ROSE, but many patient-centric articles on cancer don’t discuss rapid on-site evaluation despite all the benefits of ROSE. We want to create awareness among the patients. You see in ad campaigns, “Ask your doctor about this, ask your doctor about that.” That’s the awareness that we want to bring. To do this, we hope to partner with at least four to five cancer societies or associations to develop an unbranded patient awareness campaign focused on the need to perform ROSE.
Yes, we do have KPIs. We have metrics, etc., but those are mostly product-driven metrics. We also have internal commercial KPIs because we need to have sales at the end of the day, too.
MC: Knowing the importance of ROSE, your goal is to educate patients and get them to ask their physicians if it’s right for them — flipping the script, if you will. Certainly there isn’t any harm in patients asking their doctor about ROSE. If ROSE is something that reduces patient visits, reduces visits into the hospital for diagnostic procedures, saves money for the insurer, hospital and patient, and saves time for hospital staff, then this sounds like a wonderful thing that you’re doing. So what is the future of ROSE? What do you see coming with ROSE in the context of the broader healthcare environment?
HS: We see rapid on-site evaluation being used more and more in these procedures. Let me give you an example of the patient journey, and then I’ll explain why I expect ROSE procedures to increase exponentially in the near future. Currently, a patient gets screened for lung cancer. They get a CT and, according to current guidelines, anyone over the age of 50 and a regular smoker needs to get an FNA of the lung to confirm the CT results. Today, only 5% of the eligible population is getting screened for lung cancer. Compare this to colonoscopy, when, even with the challenges associated with it, close to 70-80% of the eligible population get a colonoscopy. There are a variety of reasons why only 5% of the eligible population goes through CT for lung cancer screening. The US federal government and cancer societies are constantly encouraging patients to go in for these kinds of screenings. Of the patients who get screened, a proportion will have a tumor in their lung, and then these people must have FNA procedures. So right now, a small proportion of the eligible population gets screened, and a smaller proportion gets FNAs, and only a fraction of them go through the rapid on-site evaluation process. If this 5% were to increase to 10%, there would be a significant increase in FNA procedures performed, and that will require ROSE. If this were to increase close to 15-fold, then you’ll see a massive increase in the need for ROSE procedures. We foresee the number of ROSE procedures continuing to expand for years to come.
We also foresee that ROSE may actually morph into what we could call “same day diagnosis to treatment.” Many of these technologies are improving every day. With FNA procedures specifically, those technologies have improved so much that when the physician finds a cancerous tumor, they can ablate the tumor using microwave or thermal ablation. This is what may happen in the next 2, 3, or 4 years, when the patient doesn’t have to come back multiple times to the hospital – once for diagnosis, next for treatment – and it may all happen on the same day. In this scenario, ROSE would play a critical role and not just for adequacy analysis. It could be used for cancer diagnosis where the sample is obtained from the patient, the physician looks at it immediately to make a diagnosis, and once confirmed to be cancer, the physician could ablate it immediately. So, to answer your question, we foresee two things. First is the expansion of ROSE itself, and the second is the morphing of ROSE from general adequacy assessment to something more, a diagnostic assessment.
MC: It is clear how the solution that ASP Health has put together would help address the increase in ROSE procedures while de-stressing the whole process. This could then aid in diagnosis followed by an interventional procedure, all on the same day. That sounds very promising!
Those are all the questions I have. Thank you for your time, and thank you for sharing your background and information and your perspectives on ROSE and what may happen with rapid on-site evaluation in the future.
Learn more about ASP Health at https://asp-health.com/
Learn more about the RC500 Remote Collaboration System at https://www.accu-scope.com/product/rc500/